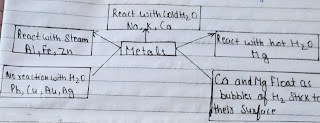
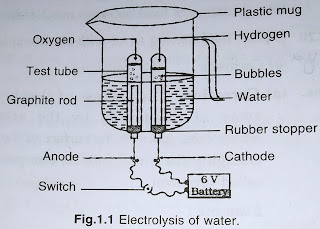
cbse notes for class 10th science ||Science class-10th notes chemical reaction and Equations
chemical reactions and equations →The process in which new substances with new properties are formed from one or more substance is called chemical reaction. •The substances which take part in chemical reaction are called reactants. •The substances which are formed in a chemical reaction are called Products. Examples:- (I)Digestion of food (II)Respiration (III)Rusting of iron (IV)Burning of magnesium ribbon (V)Formation of curd Chemical reaction involves: •change in state •change in colour •change in temperature •Evolution of gas →chemical Equation •A chemical reation can be represented by chemical equation.It involves used of symbol of elements or chemical formula of reactants and product with mention of physical state. •The necessary conditions such as temperature, pressure or any catalyst should be written on arrow between reactant and products.eg magnesium is burnt in air to form magnesium oxide. Mg +o²→ Mgo balancing chemical equations • law of conservation of