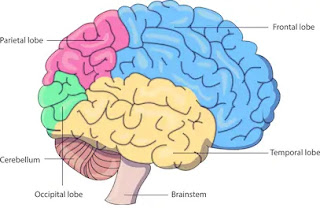
Cbse notes for class 10th Science||Notes Metals and non-metals chapter-3 school
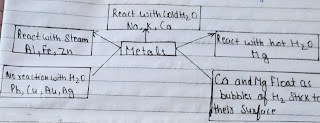
Metal and Non Metal •Elements can be Classified as metals and non-metals on the basis of their properties. •Example of some metals are: Iron(Fe), Aluminium(Al) Silver(Ag), Copper(Cu) •Example of some non-metals are: Hydrogen(H), Nitrogen(N), Sulphure(S) ,Oxygen(O) difference between metal and non metal Physical properties of metals 1.lustre: Metals have shining surface. 2.Hardness: They are generally hard.Except sodium,lithium and Potassium which are soft and can be cut with knife. 3.state: Exist as solids.Except Mercury. 4.Malleability: Metals can be beaten into thin sheets.gold and silver are the most malleable metals. 5.Ductility: Metals can be drawn into thin wires. 6.Conductor of heat and electricity: Metals are good conductors of heat and electricity.Silver(Ag) and copper(Cu):Best conductors of heat.lead(Pb), mercury(Hg)poor conductor of heat. 7.Density: Generally have high density and high melting point.Except sodium and Potassium. 8.Sonorous: Metals produce a sound on strikin